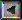The uncertainty in the scattering angle (delta theta) is approximately the vertical uncertainty (delta p sub y) in momentum divided by the horizontal momentum (p sub o) of the beam. Wave particle duality tells us that the horizontal momentum is Planck's constant over the horizontal wavelength of the beam. Putting it all together, Planck's constant cancels out, and we get the fourth equation which is the classical diffraction theory rule of thumb that the angular diffractive spread of the beam is well approximated by the horizontal wave length divided by the vertical width of the slit.
When the wave length is small compared to the slit width we have geometric optics in which light rays move along straight lines. The Schrodinger equation reduces to Newton's mechanics in an analogous limit where the wavelength of the particle is small compared to the regions over which there is significant variation in the potential energy of the particle.